Here we are going to help you guys with one of the subjects which are not very difficult but if the topic is not clear then the students will be stuck and find problems in solving the solutions related to such topics.
Oxygen Valence Electrons
Valence Electrons: 2s 2 p 4 Electron Dot Model. Oxygen - O (EnvironmentalChemistry.com)- Comprehensive information for the element Oxygen - O is provided. Carbon monoxide is apparently displaced from cytochrome A3 oxidase by a surfeit of oxygen, and electron transport is restored. Lipid peroxidation in the brain is halted. If the patient is given oxygen to breathe at 3 ATA, the half-time for carbon monoxide elimination from the blood is reduced to 23 min. Since an oxygen atom has six valence electrons and wants eight, it uses two of its six valance electrons for bonding. The remaining four exist as lone pairs. Even though oxygen has six valence electrons, it is unable to form six covalent bonds. If oxygen formed six covalent bonds, then it would share a total of 12 electrons.


What is the Valence of Oxygen in Water?
- Valence electrons are a name for how atoms interact when forming chemical bonds. There are not really electrons that are specifically valence electrons. Oxygen is a good example of this. It has 8 electrons (because it has 8 protons). 2 of the electrons are in the 1s shell, and that shell is.
- Hence, each of F atoms will attract one electron from oxygen i.e. F will show -1 oxidation state and O will show +2 oxidation state. Whereas, in the case of Na 2 O, oxygen is highly electronegative than sodium atom. So oxygen will attract two electrons from each sodium atom showing -2 oxidation state and Na will have +1 oxidation state.
Periodic Table Charges of Elements
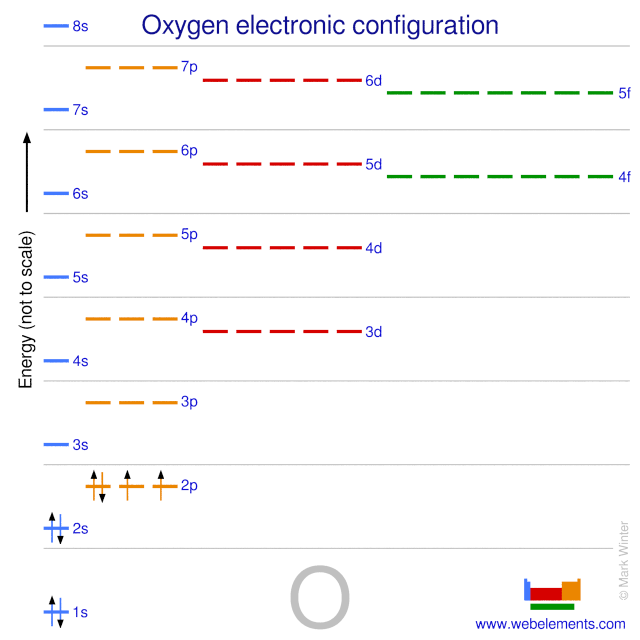
Oxygen Electron Configuration
How many valence electrons does oxygen have?
1 Answer
Oxygen has 6 valence electrons.
How Many Valence Electrons In O2
A way to remember this is to note that it is in column 16 of the periodic table. For the representative elements (columns 1, 2, 13-18), the digit in the units place of the column number is the same as the number of valence electrons. Elements in column 1 have one valence electrons, elements in column 13 have 3 valence electrons, etc.
Oxygen Valence Electrons Configuration
The 2 electrons on the top represent the
Related questions